

Harnessing the Potential of UiO Series Metal-Organic
Frameworks (MOFs) and Composites
Globally, heavy metal pollution is a major issue, particularly in developing nations where farming and industry discharge metal ions into sewage and water supplies . According to (Ahmadijokani, 2024) these hazardous toxic metals, which include Cd, As, Cr, Cu, Pb, and Hg, can accumulate in both humans and animals and pose major risks to both the environment and human health.(Mehdi & Aravamudan, 2024)
Eliminating these contaminants from wastewater is essential. Conventional techniques can lead to additional environmental problems since they are frequently costly and intricate (Tang et al., 2024) Recently, adsorption has become a cost-effective and strong approach to remove heavy metal ions from water, making it a green alternative The transport of contaminants to materials that can absorb them is improved by this strategy.
Scientists are becoming more interested in metal-organic frameworks (MOFs) because to its special qualities, which include stability, high porosity, and a huge surface area (Zhao et al., 2021). UiO-MOFs, notably UiO-66, UiO-67, UiO-68, and UiO-69, have exhibited stability and active binding sites, making them attractive for removing heavy metal ions.
The active binding sites in the organic ligands make UiO-MOFs unique. According to the hardsoft-acid-base principle, they can be changed with certain functional groups to increase sites for heavy metal ion adsorption. Enhancing UiO-MOFs’ adaptability and dependability for environmental applications is the aim.

The issue of heavy metal pollution has spread throughout the environment and has serious repercussions for both human health and ecosystems. These metals, which include lead, mercury, cadmium, and arsenic, are frequently absorbed into the soil, water, and atmosphere as a result of industrial processes, inappropriate waste management, and natural events.
The environment is now seeing an increased amount of heavy metal emission due to industrialization and growing urbanization. These contaminants are dispersed through mining, manufacturing, and agricultural practices, which causes them to accumulate in different environmental compartments.
Because heavy metals are persistent, they endanger ecosystems and the delicate balance of natural processes, which in turn threatens biodiversity. Furthermore, as these metals find their way into our food supply, they run the risk of bioaccumulating in the food chain and endangering human health.
The need to address heavy metal contamination stems from its complex effects. Because of their recognized toxicity, heavy metals can harm living things even at very low concentrations. Numerous health problems, such as neurological diseases, developmental abnormalities, and carcinogenic consequences, have been related to human exposure to heavy metals.(Zhao et al., 2021)
Moreover, water quality is compromised by heavy metal pollution, which has an impact on aquatic life and ecosystems. Pollution of the soil reduces agricultural yield and can introduce metals into the food we grow.
Given the seriousness of these effects, it is imperative to develop long-term, practical solutions to reduce heavy metal contamination. Innovative approaches are essential since conventional methods frequently fall short in terms of efficiency, cost-effectiveness, and environmental impact.
The UiO series MOFs are reviewed in this review as novel materials that effectively remove heavy metal ions from the environment. Because of their adaptability, stability, and biocompatibility, UiO-MOFs and modified UiO-MOFs have shown to be successful adsorbents. The review includes a thorough explanation of how UiO-MOFs absorb heavy metal ions as well as information on how these materials are created and where they are employed. The objective is to provide insightful information to scientists investigating long-term remedies for heavy metal-contaminated wastewater.
A Brief Synopsis A careful balancing act between experimental property research and real-world applications is required to create UiO-MOFs. These three-dimensional porous materials (UiO-64, UiO-66, UiO-67, UiO-68, UiO-69, and their derivatives) with Zr4+ and dicarboxylic acid ligands preserve a reticular structure that is invariant to changes in the length of the ligands. Zr (IV)-based MOFs with Zr6O4(OH)4 as the secondary building unit (SBU) have a cubic close-packed (CCP) structure, as demonstrated by UiO-66, UiO-67, UiO-68, and UiO-69. Together with twelve bridge ligands, this SBU forms a strong three-dimensional structure with eight tetrahedral corner cages and an octahedral central hole cage.
The strength of Zr–O bonding in UiO-MOFs is highlighted by theoretical values for pore volumes and specific surface areas. The focus of UiO-MOF synthesis has turned to functionalization and ligand length modifications, providing control over pore size, morphology, and physical/chemical characteristics. The synthesis method preserves the crystalline and topological structures of UiO-MOFs and their functional composites by depending on interaction forces like metal bonds, hydrogen bonds, coordination bonds, and π– π interactions.(Yang et al., 2022) Initial pore properties are determined by metal ions and linkers, whereas solvent selection, pH, and reaction temperature affect structural details. A range of synthesis tactics, including solvent-thermal, microwave, volatilization, diffusion, ultrasonic, and mechanical stirring processes, provide flexibility customized to particular needs in the production of UiO-MOFs.
A careful balance between experimental research and real-world applications must be struck while creating UiO-MOFs. These three-dimensional porous materials (UiO-64, UiO-66, UiO-67, UiO-68, UiO-69, and its variants) with Zr4+ and dicarboxylic acid ligands preserve a reticular structure that is invariant to ligand length alterations.
UiO-66, UiO-67, UiO-68, and UiO-69 are examples of Zr (IV)-based MOFs that demonstrate a cubic close-packed (CCP) structure with Zr6O4(OH)4 serving as the secondary building unit (SBU). This SBU creates a strong three-dimensional structure with eight tetrahedral corner cages and an octahedral center hole cage, coordinating with twelve bridge ligands. Theoretical values for pore volumes and specific surface areas underline the strong character of Zr–O bonding inside UiO-MOFs.(Rasheed et al., 2020)
UiO-MOF synthesis has developed to prioritize functionalization and ligand length modifications, offering control over morphology, pore size, and physical/chemical characteristics. Utilizing interaction forces such as metal bonds, π–π interactions, coordination bonds, and hydrogen bonds, the synthesis procedure guarantees the retention of crystalline and topological structures in UiO-MOFs and their functional composites.
Metal ions and linkers control initial pore properties, while reaction temperature, pH, and solvent selection shape structural details. A range of synthesis methodologies, such as solventthermal, microwave, volatilization, diffusion, ultrasonic, and mechanical stirring processes, provide customized flexibility to fulfill particular needs for creating UiO-MOFs.(Chueh et al., 2019).
In UiO-MOF synthesis, the solvent-thermal technique leads the way by precisely addressing problems caused by the inability to dissolve some reactants at room temperature. This process is excellent at generating morphology at the nanoscale, guaranteeing high yields, and obtaining perfect crystals. The features of solvents such as viscosity, polarity, dielectric constant, and functional group help to refine the synthesis routes of UiO-MOF, leading to a variety of product morphologies and sizes.
Because of its ease of use and effectiveness, the solvent-thermal technique yields better morphology and improved crystal performance.(Lu et al., 2017) By dissolving ZrCl4 and NH2BDC in DMF and treating them with solvothermal treatment at 120°C for 24 hours, Feng et al. demonstrated the synthesis of UiO-66-NH2 crystals. This resulted in a perfect cubic structure intended for the removal of Cr (III) and Cr (VI) (Fig. S1A) (Feng et al., 2019). Lu et al. described the synthesis of UiO-66 in detail. They did this by combining terephthalic acid (H2BDC) and zirconium tetrachloride salt, then used a similar procedure (Fig. S1B) to dissolve the mixture in N, N′-dimethylformamide (DMF). Another strategy was to add coordinationfree COOH groups to the UiO-66 framework using a ligand combination of trimellitic and terephthalic acids.(Bonneau et al., 2020)
The solvent-thermal approach has been shown to be a dependable and favored technology for producing UiO-MOFs due to its precise homogeneity, high crystalline, and good topological morphology in its products.(Feng et al., 2019)
The synthesis of MOFs is predicated on the practical application and experimental property study. UiO-MOFs are made of dicarboxylic acid ligands and Zr4+, making them threedimensional porous materials. UiO-64, UiO-66, UiO-67, UiO-68, UiO-69, and their derivatives all share the same reticular structure, despite the fact that their ligands varied in length. However, the thermal stability of UiO-MOFs remains unaffected by ligand changes. Similar cubic close-packed (CCP) structures are generated by Zr(IV)-based MOFs with larger versions. including (Zr6O4(OH)4(BDC)6 for UiO-66; Zr6O4(OH)4(BPDC)6 for UiO-67;
Zr6O4(OH)4(TPDC)6 for UiO-68, and Zr6O4(OH)4(2,6-NDC)6 for UiO-69.(Butova et al., 2016).
MOFs have appealing physicochemical characteristics that make them appropriate for a variety of uses, including the treatment of water. There are numerous synthetic methods for creating MOFs. They fall into two categories: conventional and non-traditional techniques. One of the traditional techniques is hydrothermal therapy.as well as solvothermal methods. While the methods that are regarded as non-conventional include microwave-assisted, electrochemical, ionothermal, and mechanochemical.(Q. Wang et al., 2020).
A productive method for producing UiO-MOFs that offers moderate conditions and quick synthesis durations is electrochemical technology (Al-Kutubi et al., 2015). This approach does not require conductive metal salts because metal ions are produced by electrochemical processes in solutions containing organic ligands and electrolytes. By using this method, the synthesis system is spared the effects of anions. Significantly shorter reaction times, autonomous control over the reaction, and real-time monitoring via an electrochemical workstation are all made possible by electrochemistry, which also improves our comprehension of the reaction mechanism.
Wei et al. (2019) used metal Zr as the metal source in an electrochemical process that produced an ultra-stable UiO-66-NH2 material at room temperature and pressure. Using zirconium foil as a metal source, Saleem et al. (2016) presented an electrochemical film deposition technique based on anode and cathode electrochemical film deposition of UiO-66. This electrochemical method’s ability to deposit patterns enables UiO-66 to be directly integrated as an adsorbent film into a tiny sorbent trap for online analysis (Fig. S2). Using pre-synthesized UiO-67 film as an electrocatalyst for water oxidation, Lin et al. (2017) investigated the production of RuUiO-67 on a conductive surface for electrochemical water oxidation via electrode location or drop-casting. electrochemical technology provides a new way to synthesize UiO-MOFs, allows for controlled synthesis rates, and allows real-time reaction monitoring.
In recent years, the synthesis of UiO-MOFs has seen the application of numerous methodologies, including mechanical and ultrasonic approaches. The first step in the mechanochemical synthesis process is to combine organic ligands and metal salts with a little amount of solvent. The material for the MOFs is then prepared by grinding it, either in an agate mortar or a ball mill. Thanks to the use of mechanical energy, this environmentally friendly technique allows for the synthesis of MOFs at high yields while requiring less solvent.
On the other hand, ultrasonic synthesis uses the solvent’s acoustic cavities that are created by ultrasonic waves. As a result, the solution experiences localized high temperatures (about 5000 K) and pressures (about 1000 ATM), which increase the reactants’ activity. Both approaches have disadvantages despite their benefits, such as significant energy consumption, lengthy synthesis times, complex procedures, and strict instrument requirements.The main focus of current UiO-MOF synthesis research efforts is to identify processes that are mild and ecologically benign in order to overcome the drawbacks of the previously listed approaches.
UiO-MOF composites are produced via a variety of processes, including linker enlargement, dopant modification, post-synthesis methods (PSMs), and functional nanoparticle (NP) encapsulation. As shown in Figure 2, these composite materials are useful in environmental sample applications while preserving the original topological features. The diverse molecular roles and architectures of different ligands and inorganic nodes give rise to the distinct chemical and physical properties displayed by modified MOFs. As a result, the functionalization of MOFs has become dependent on the deliberate design of certain ligands and the doping of metal ions within the framework.
To synthesize functionalized MOFs, one can modify the length of the ligands and add functional groups to the ligands or the secondary building unit (SBUs, which are metal-oxygen clusters or single metal ions) to control the pore size and functionality. What’s important is that these changes take place without changing UiO-MOFs’ topological structure. Chemical properties of UiO-MOFs have gained significant attention, particularly in post-synthesis modifications, crystal size tuning, and functionalization of pores and outer surfaces to enhance adsorption efficiency and selectivity (Yu et al., 2019; Mukhopadhyay et al., 2019; Donnadio et al., 2017; Lv et al., 2016).
The terms “post-synthesis methods” (PSMs) include post-synthesis deprotection (PSD), dative post-synthesis methods (dative PSMs), and covalent post-synthesis methods (covalent PSMs) (see Fig. 3). (Cohen, 2012). As of right now, covalent PSMs have shown to be a powerful and adaptable method for adding different chemical groups to MOFs. Examples include the production of novel functionalized frameworks by reticular chemistry, such as amino, bromo, nitro, carboxylic-acid, and polyethyleneimine-functionalized UiO-66 (Sarker et al., 2018; Zhu et al., 2019; Garibay and Cohen, 2010). When such ligands are added, UiO-MOFs’ crystal structure and topology are preserved but their physical and chemical characteristics are changed, producing a variety of particular surface areas. UiO-MOFs-Br and UiO-MOFs-NO2, on the other hand, have lower specific surface areas than UiO-MOFs due to the reduction of free space within the UiO-MOFs frame cage caused by the insertion of larger-volume and mass ligands like -Br and -NO2. Although NH2 has a minor effect on the cavities of UiO-MOFs-NH2, its specific surface areas are nevertheless comparable to those of UiO-MOFs. According to Lou and Liu (2019), UiO-66-Br and UiO-66 exhibit stability above 450 ℃, whereas UiO-66-NH2 and UiO-66-NO2 start to break down at temperatures lower than 350 ℃.
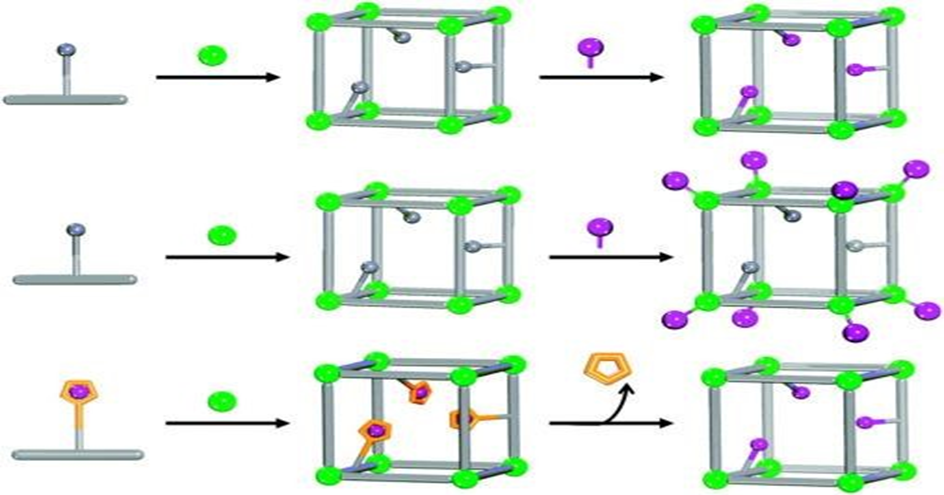
cEmbedding functional nanoparticles (NPs) into the MOF architecture is an alternate method of functionalizing UiO-MOFs that yields sophisticated composites with a variety of characteristics, including luminescence, magnetism, and catalysis (Lei et al., 2014). UiO-66 derivatives with functional groups such -COOH, -NH2, -OH, and -SO3H have better catalytic performance, can adsorb more hazardous substances, and can make it easier to synthesize luminous materials (Zhang et al., 2019). Interestingly, the incorporation of lanthanide ion (Ln3+) systems into metal-organic frameworks (MOFs) provides a distinct platform for luminous materials by taking advantage of their well-defined environment for crystal-state luminescent clusters (Yan, 2017).
It has been shown that adding nanoparticles to UiO-MOFs can improve their adsorption and separation properties. An active field of study is the combination of diverse nanoparticles and high-performance UiO-MOFs for the elimination of heavy metal ions. By utilizing their distinct qualities, composites containing MOFs and graphene or graphene oxide not only preserve the inherent qualities of each material but also provide new synergistic effects (Lu et al., 2019). Fang et al. (2019), for example, created an electrochemical sensor that uses reduced graphene oxide (RGO) and UiO-66-NH2 composites as electrode materials for Cu2+ and ciprofloxacin (Cip) complexation processes. A unique three-component Z-scheme system was presented by Meng et al., which combined O-ZnO and UiO-66-NH2 as photocatalysts with RGO as an electronic medium. O-ZnO/RGO/UiO-66-NH2 heterostructure that resulted showed increased photocatalytic activity in CO2 reduction (Meng et al., 2019). In order to get better adsorption capacity, the integration of magnetic nonmaterial into UiOMOFUiO-MOF core-shell structures has also been investigated. For example, the significant specific surface area of Fe3O4 increases the adsorption capacity when it is integrated into coreshell frameworks. But for magnetic UiO-MOF composites to be used in real applications, problems like aggregation—which are caused by excess surface energy and magnetism—must be resolved. For these materials to become more useful, it is imperative that this paradox be resolved.
MOFs can be functionalized, i.e., their pore sizes can be expanded. Ye et al. (2019) studied the catalyst [(CH2COOH)2IM]HSO4@H-UiO-66] by using a bidentate coordination technique between a –COO– group of [(CH2COOH) 2IM] HSO4 and two unsaturated Zr ions. This approach successfully expanded the pore sizes and surface areas. More broadly, complexes with catalytically active Ir, Re, and Ru with dicarboxylic acid functional groups have been successfully integrated into a robust and porous Zr6O4(OH)4(BPDC)6 (UiO-67) framework through a mix-and-match synthesis strategy with expanded organic ligands (Wang et al., 2011). This synthesis approach works well in expanding UiO-MOF pore sizes.
MOFs can become functionalized through the modification of organic linkers, particularly through the increase of pore diameters. Ye et al. (2019) utilized a bidentate coordination approach to deploy the catalyst [(CH2COOH)2IM]HSO4@H-UiO-66. This involved a –COO– group of [(CH2COOH) 2IM] HSO4 and two unsaturated Zr ions. This technique successfully expanded the surface areas and pore diameters. More generally, a mixand-match synthesis strategy with expanded organic ligands has allowed for the successful integration of complexes containing catalytically active Ir, Re, and Ru with dicarboxylic acid functional groups into a robust and porous Zr6O4(OH)4(BPDC)6 (UiO-67) framework (Wang et al., 2011).
This synthesis technique proves successful in widening UiO-MOF pore diameters, permitting enhanced diffusion of metal ions within the MOFs (Zhao et al., 2020a, Zhao et al., 2020b).
Long-chain organic ligand molecules have benefits, but it’s important to remember that they’re typically more expensive, which may restrict their commercial availability. Regarding the structural stability of the synthesized UiO-MOFs, the length of the organic ligands also needs to be taken into account because too long of a length could cause pore collapse or interpenetration.
The effectiveness of MOFs to remove heavy metal ions from wastewater treatment depends on the materials’ strong adsorption capacity and structural stability. Strong Zr–O bonds and a large number of coordinatively unsaturated Zr4+ sites (CUS) make UiO–MOFs useful for effective mass transfer and adsorption. The addition of longer organic linkers throughout the synthesis process augments the number of adsorption sites in the resulting materials (Furukawa et al., 2013).
One particular UiO-MOF, UiO-66, has exceptional chemical stability; it remains resilient across a range of solvents and pH values. It can therefore be relied upon as an adsorbent in both acidic and alkaline conditions.
The strength of the coordination bond between the organic ligands and the adsorbent’s performance determines the stability. UiO-66, with its shorter ligands’ chains and powerful coordinated bonds, displays broader applicability compared to other UiO series materials (Li et al., 2017a, Li et al., 2017b). To improve stability, more research entails altering UiO-MOFs with various ligands, such as those that include porphyrin rings (KO Et Al., 2015).
As novel materials, UiO-MOFs have excellent adsorption capability, cost-effectiveness, environmental friendliness, and accessibility for the removal of heavy metal ions in wastewater treatment. Key features that distinguish UiO-MOFs from traditional adsorbents like activated carbon and zeolites include greater adsorption capacity and efficiency, attributable to strong coordinated bonds or magnetic properties. Compared to traditional adsorbent techniques, the adsorption mechanisms used by UiO-MOFs for the removal of heavy metal ions are thought to be more rigorously scientifically supported. In order to produce UiO-MOFs on a wide scale, research is currently focused on streamlining the synthesis process, boosting product yield, and implementing economical or ecologically friendly reagents. Finding high-efficiency MOFs for heavy metal removal that are suited to environmental concerns is the goal of this research.
In response to the increasing need for continuous monitoring and effective mitigation of organic and inorganic pollutants, a boom in research has studied Metal-Organic Frameworks (MOFs), especially UiO-MOFs and their composites, as promising improved sorbents for heavy metal ion removal. The UiO series’ leading candidate, UiO-66, has become well-known for its ultra-low density, precise cavity structure, mechanical stability, and thermal robustness.
This review includes pivotal studies on UiO-MOF synthesis and their usage as efficient sorbents. Their efficacy is highlighted by the agreement of adsorption properties and mechanisms with pseudo-second-order models, Freundlich isotherms, and Langmuir isotherms. Studies on thermodynamics support the idea that metal ion adsorption occurs spontaneously. Nevertheless, difficulties still exist, calling for the creation of environmentally friendly synthesis techniques, investigation of industrial uses, comprehension of MOF functions, and cost-effectiveness considerations. The creation of novel MOFs with improved stability, selectivity, adsorption characteristics, and reusability will be essential to future advancements in separation science. The focus is on streamlining MOF preparation while preserving affordability and environmental friendliness. UiO-MOFs continue to hold great promise for sustainable development, especially when it comes to eliminating extremely hazardous organic metals. However, the move from lab research to commercial applications necessitates resolving financial limitations, sorbent regeneration, and careful chemical usage. By addressing these issues and investigating new avenues for study, MOF chemistry will continue to flourish and have a bright future.
We certify that there isn’t a conflict of interest involving the work that was turned in. The study has the consent of all co-authors and pertinent authorities and is original and not being considered elsewhere.
The natural environment and human health have both been endangered by heavy metal pollution, hence it is crucial to remove these hazardous contaminants from a variety of complicated substrates. Adsorption remains one of the most successful methods to date. Metalorganic frameworks (MOFs) are porous crystalline materials that are made up of coordination bonds between metal ions or metal clusters and organic ligands. Recently, environmental analytical chemistry has shown a great deal of interest in these materials because of their high surface area, porosity, and outstanding chemical/thermal stability. The applications of UiO series MOFs and their composites as emergent MOFs, which have been successfully exploited as novel adsorption materials for the adsorption and removal of various heavy metal ions from a variety of environmental samples, were the primary emphasis of this review’s current findings. Furthermore, a detailed explanation of UiO-MOFs and their composites, including the synthesis processes and uses of these materials in the removal of heavy metal ions, was given. The adsorption isotherms equation, adsorption thermodynamics, and kinetics were also covered, as well as the adsorption properties and mechanism of UiO-MOFs as solid sorbents for heavy metal ions. To this purpose, the developing trends of MOF-based composites for the removal of heavy metal ions were also prospected. This review will offer fresh perspectives for investigating the adsorption mechanism of heavy metal ions on sorbents and creating highperforming media for effective pollution removal from wastewater.
Solvent-thermal synthesis is the most widely used technique for creating UiO-MOFs. This method resolves the issue of certain reactants being difficult to dissolve at room temperature by producing nanoscale morphology, high product yields, and flawless crystallinity (Ahmed et al., 2019) Furthermore, the synthesis pathways of UiO-MOFs are proportionally improved due to the differences in the polarity, dielectric constant, functional group, and viscosity of the solvent used in the process; thus, the size and morphology of the products are likewise different. Solvent-thermal analysis, the most straightforward and effective technique, was frequently employed to enhance crystal performance and acquire superior crystal morphology. Feng et al. reported that ZrCl4 and NH2-BDC were dissolved in DMF, and then placed in Teflon-lined stainless autoclave for solvothermal at 120 °C for 24 h to get a perfect cubic structure of UiO66-NH2 crystal for the removal of Cr (Ⅲ) and Cr (VI) (Fig. S1A) (Feng et al., 2019). Lu et al. reported the synthesis of UiO-66 as combining terephthalic acid (H2BDC) with zirconium tetrachloride salt, followed by the above-mentioned dissolution in N, N′-dimethylformamide (DMF) (Fig. S1B) (Lu et al., 2017). Similarly, coordination-free-COOH groups were prepared on the UiO-66 framework using a ligand combination consisting of trimellitic and terephthalic acids (Ahmed et al., 2019). As a result, the product created using the solvothermal process has a topological morphology that is satisfactory, uniform size, and high crystallinity.
Compared with solvent-thermal technology, the microwave is dipolar molecule vibration to generate heat, therefore more efficient and faster than thermal transport, which is regarded to be one of the key reasons for microwave-assisted rapid synthesis of UiO-MOFs. The benefits of microwave irradiation are quick and even heating, reduced time, low energy use, and no pollution. Furthermore, by varying the metal-ligand ratio and crystallization period, it may efficiently modify the crystal size. According to Vakili et al., the reaction time was reduced to two to three quarters of an hour when the UiO-67 was made utilizing benzoic acid and hydrochloric acid as the regulators under microwave irradiation.
(Zhang et al., 2020) The benefits of microwave irradiation are quick and even heating, reduced time, low energy use, and no pollution. Furthermore, by varying the metal-ligand ratio and crystallization period, it may efficiently modify the crystal size. Vakili et al. stated that the UiO-67 was made utilizing benzoic acid and hydrochloric acid as the regulator under the microwave irradiation, which decreased the reaction time to 2–2.5 h. UiO-67/CdS composites were created using the microwave-solvothermal technique, which involved a microwave heating reaction at 160 °C for 30 minutes at an 800 W heating output. Zr-MOFs were made by heating in a microwave oven for 5 min, which dramatically decreased the reaction time and boosted the reaction rate(K. Wang et al., 2018).The synthesis efficiency of goods can be significantly increased using the microwave irradiation approach.
Another method for creating UiO-MOFs with short synthesis times and benign synthesis conditions is electrochemical technology (Al‐Kutubi et al., 2015) This approach avoids the impact of anions on the synthesis system by producing metal ions through electrochemical reactions in solutions containing organic ligands and electrolytes without the need for conductive metal salts. The reaction can be independently controlled and the reaction time can be effectively reduced by electrochemistry. Furthermore, real-time recording of the reaction process using the electrochemical workstation facilitates a deeper comprehension of the reaction mechanism. According to (Wei et al., 2019) metal Zr was used as the metal source in an electrochemical process that produced an ultra-stable UiO-66-NH2 material at room temperature and pressure. Saleem et al. (2016) created an electrochemical film deposition technology based on the anode and cathode electrochemical film deposition of UiO-66, using zirconium foil as a metal source.
UiO-66 could be directly integrated as an adsorbent film into a small sorbent trap for online analysis thanks to the electrochemical method’s patterned deposition capability. Pre-synthesised UiO-67 film was utilized as an electrocatalyst for water oxidation in Lin et al. (2017)’s investigation into the synthesis of Ru-UiO-67 on a conductive surface and its propensity for electrochemical water oxidation via electrodeposition or dropcasting. As a result, electrochemical technology can regulate the rate of synthesis. Additionally, the electrochemical synthesis method offers a novel approach to the synthesis of UiO-MOFs as well as the possibility of direct real-time reaction.
Recently, UiO-MOFs have been synthesized via mechanical, ultrasonic, and other techniques. The mechanochemical process involves the first mixing of metal salts and organic ligands, then a little amount of solvent, and then grinding in an agate mortar or ball mill to prepare the MOFs material. In the presence of mechanical energy, it is an environmentally benign process that enables the synthesis of MOFs with high yields and little solvent. The goal of ultrasonic synthesis is to continually create high local temperatures (about 5000 K) and pressures (approximately 1000 ATM) in the solution by using the acoustic cavity created by the ultrasonic wave in the solvent. This can increase the reactant’s activity.
These techniques do have certain drawbacks, like high energy consumption, lengthy synthesis times, intricate synthesis procedures, and stringent equipment requirements. Thus, the primary focus of UiO-MOFs synthesis research is to identify some synthesis techniques that require gentle and hygienic settings.
By varying the ligand length and functionalizing the ligand or secondary building unit (SBUs, which stand for single metal ions or metal-oxygen clusters) without altering the topological structure, the pore size and function can be changed during the synthesis of functionalized MOFs. In addition, the chemical properties of UiO-MOFs have gotten more and more attention, especially in the fields of post-synthesis modification, crystal size tuning, and functionalization of pores and the outer surface for increasing the efficiency and selectivity of adsorption (Yu et al., 2019, Mukhopadhyay et al., 2019, Donnadio et al., 2017, Lv et al., 2016). PSMs consist of three methods: post-synthesis deprotection (PSD) (Fig. 3), dative postsynthesis method (dative PSMs), and covalent post-synthesis method (covalent PSMs) (Cohen, 2012).
Currently, covalent PSMs have shown to be an effective and adaptable way to add different kinds of chemical groups to MOFs. For instance, reticular chemistry has been used to synthesis amino, bromo, nitro, carboxylic-acid, and polyethyleneimine-functionalized UiO-66, resulting in the creation of novel functionalized frameworks by PSMs (Sarker et al., 2018, Zhu et al., 2019, Garibay and Cohen, 2010).
Consequently, the addition of these ligands can alter the chemical or physical characteristics of UiO-MOFs while preserving their topology and crystal structure and displaying a variety of unique surface areas. However, the free space in the UiO-MOFs frame cage will be reduced upon the introduction of functional ligands with bigger volume and mass, such as –Br and –NO2, leading to lower specific surface areas of UiO-MOFs-Br and UiO-MOFs-NO2 than UiO-MOFs. While NH2 has a minor impact on the cavities of UiO-MOFs-NH2, the particular surface areas can be maintained in a manner comparable to that of UiO-MOFs. For instance, UiO-66-Br and UiO-66 can both remain stable at 450 ℃, but UiO-66-NH2 and UiO-66-NO2 start to break down around 350 ℃. Liu and Lou (2019).
Combining functional NPs with the MOF architecture is another method of functionalization that produces sophisticated composites with numerous features, such as luminescence, magnetism, and catalysis (Lei et al., 2014). UiO-66 with −COOH, −NH2, −OH, and −SO3H, for instance, can enhance the catalytic performance of materials, the adsorption performance of hazardous compounds, and the synthesis of luminous materials as derivatives of UiO-MOFs (Zhang et al., 2019).
For instance, because of their predictable structure and well-defined environment for luminous clusters of crystal state, MOFs, such as the Lanthanide Ion (Ln3+) system, offer a special platform for luminescent materials (Yan, 2017). The adsorption and separation capabilities of UiO-MOFs have been enhanced by the incorporation of nanoparticles; investigating the combination of high-performance UiO-MOFs and other nanoparticles to remove heavy metal ions remains a hot topic.
Composites based on MOFs and graphene or graphene oxide not only retain the inherent properties of each material but also can integrate the unique properties of the two fascinating materials and produce new synergistic effect (Lu et al., 2019). Fang et al. (2019) prepared an electrochemical sensor based on ciprofloxacin (Cip) and Cu2+ complexation reactions using UiO-66-NH2 and reduced graphene oxide (RGO) composites as electrode materials. Meng et al.
firstly constructed a new three-component Z-scheme system using UiO-66-NH2 and O–ZnO as photocatalyst. The photocatalytic activity of O-ZnO/RGO/UiO-66-NH2 heterostructure was investigated in the aforementioned report using the photocatalytic CO2 reduction method (Meng et al., 2019). Furthermore, the incorporation of magnetic nanomaterials into UiO-MOF@UiO-MOF coreshell structure materials can obtain excellent adsorption capacity; for instance, Fe3O4 is incorporated into the core-shell structure materials to further improve its adsorption capacity due to the large specific surface area.
However, these practical applications are also limited by the aggregation due to the excessive surface energy and magnetism. As a result, it is crucial and worthy of serious consideration to resolve the magnetic UiO-MOFs composite.
The main things to take into account when using MOFs to remove heavy metal ions from various types of sewage are the materials’ great adsorption capacity and excellent structural stability. Strong Zr-O bonds and more coordinatively unsaturated Zr4+ sites (CUS) are two characteristics of UiO-MOFs that are advantageous for mass transfer and adsorption. The produced materials provide more adsorption sites the longer the organic linker is utilized in the synthesis process (Furukawa et al., 2013). UiO-66 exhibits exceptional chemical stability; its skeleton is stable in strong acid/alkaline solutions and it remains stable in a variety of solvents, including water, DMF, benzene, acetone, methanol, and isopropanol (Nguyen et al., 2014, Fan et al., 2019). It is the most stable UiO-MOFs when compared to other UiO series materials (Li et al., 2017a, Li et al., 2017b). As a result, UiO-MOFs as an adsorbent can withstand protons or hydroxide ions in a variety of wastewater types and pH values. The stability of the adsorbent is based on its own performance as well as the strength of the organic ligand’s coordination bond; the longer the ligand, the more likely it is to cause elastic vibration and fracture during the adsorption and desorption process of water molecules, which will ultimately result in the collapse of the skeleton. Because UiO-66 and its composites have stronger coordinated bonds and shorter ligand chains than other UiO-MOFs, they have broader uses. Additionally, UiOMOFs may become more stable if they are modified with other ligands, such as those that include porphyrin rings (Ko et al., 2015). The environmental media claims that it is a research hotspot for choosing a type of MOFs with great effectiveness to remove heavy metals.
It is important to note that UiO-MOFs, as newly developed materials, have a satisfactory adsorption capacity to remove heavy metal ions in wastewater treatment while also being reasonably priced, ecologically acceptable, and easily accessible. Because UiO-66 and its composites have stronger coordinated bonds and shorter ligand chains than other UiO-MOFs, they have broader uses. Additionally, UiO-MOFs may become more stable if they are modified with other ligands, such as those that include porphyrin rings (Ko et al., 2015). The environmental media claims that it is a research hotspot for choosing a type of MOFs with great effectiveness to remove heavy metals.
It is important to note that UiO-MOFs, as newly developed materials, have a satisfactory adsorption capacity to remove heavy metal ions in wastewater treatment while also being reasonably priced, ecologically acceptable, and easily accessible.
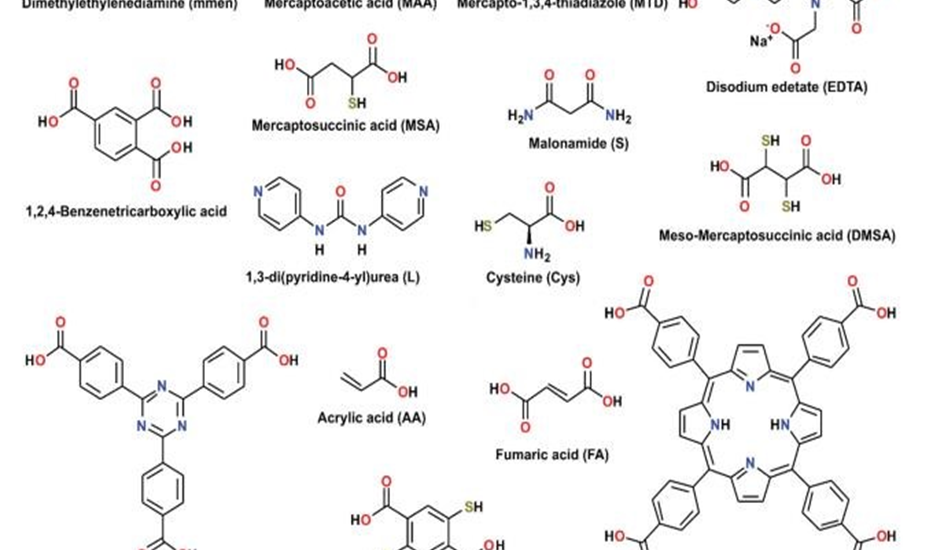
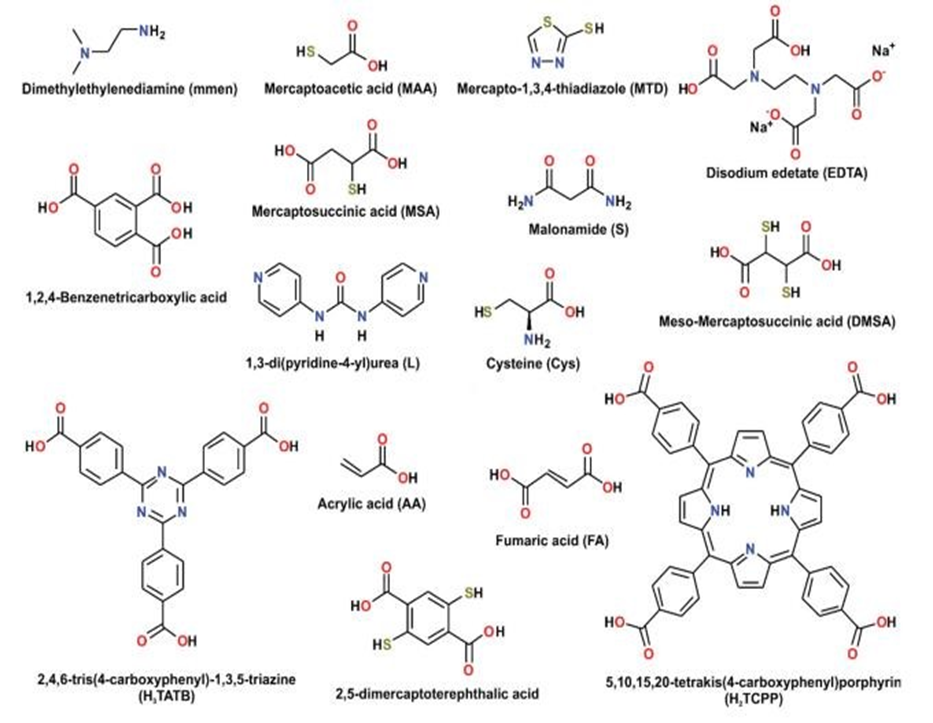
In addition to increasing a group’s active sites, functional group alteration can enhance heavy metal chelating and adsorption capabilities. In order to selectively remove Hg (II) from solution, Zhao et al. (2019) designed a novel L-cysteine-containing composite (Cys-UiO-66) by functionalizing UiO-66-NH2 with L-cysteine(Cys) (Fig. 4). At pH 5.0, the maximum adsorption capacity of Cys-UiO-66 was 350.14 mg mg g−1, compared to 112.68 mg g−1 for
UiO-66-NH2. Gadolinium ion (Gd3+) adsorption behavior on functionalized UiO-66 with −COOH and −NH2 groups (UiO-66-COOH-ED) was investigated by Ahmed et al. (2019). Comparing the original UiO-66 to the one with −COOH and −NH2 groups greatly enhanced the adsorption ability for Gd3+.Because Gd3+ coordinated with the electron-rich oxygen and nitrogen atoms of −COOH and −NH2 groups, its adsorption capacity rose by 4.9 times. According to Ding et al. (2018), Zr-based MOFs (Zr-DMBD, H2DMBD 2,5-dimercapto-1,4benzenedicarboxylic acid) functionalized with thiol groups were successfully synthesized with free-standing and accessible structures for the purpose of selectively recognizing Hg(II) in sewage. The maximum adsorption capacity of Zr-DMBD was 171.5 mg·g−1, which was roughly nine times greater than that of the original UiO-66.(Tauetsile et al., 2019)
Metal nanoparticles are easier to join with other atoms to balance surface energy because of their greater specific surface area and more unsaturated sites. This considerably improves the selectivity of metal nanoparticles. MOFs are frequently employed as the carrier to increase the materials’ adsorption capacity, and two techniques are used to load the nanoparticles: leaching reduction (Leus et al., 2015) and controlled particle encapsulation (Li et al., 2017a, Li et al., 2017b, Peng et al., 2020). Li and colleagues (2017)a, b) utilized a straightforward and expeditious technique to ascertain and eliminate Hg2+. They effectively implemented this method to treat Hg2+ in water, achieving a removal efficiency exceeding 99% through the creation of Pt NPs@UiO-66-NH2 composites, Pt NPs possessing elevated peroxidation-like activity and Hg2+-induced inhibition.(Duru et al., 2016)
Through post-synthetic modification (PSM), Zhang et al. (2019) synthesized UiO-66-type MOFs luminous materials doped with lanthanide metal element Eu3+ that are specifically able to identify Hg2+ in aqueous solution. There is a lot of promise for biosensor, imaging, and environmental analysis with these new lanthanide metal composites.(Li et al., 2018).
Selective host-guest interactions result from the creation of recognition sites on the MOF surface by the presence of certain moieties. Over the last few years, MOF customization
using organic characteristics suited to a task has emerged as a popular method for extracting particular metal ions from a matrix. The functionalities with amine, thiol, carboxylate, and hydroxyl groups are the most often employed ones. According to Pearson HSAB theory, the ligands with O, N, and S as the donors might be categorized as hard, hard to borderline, and soft bases, respectively.(Neyestani et al., 2017)
Therefore, modified MOF containing either N or S-donors can more effectively adsorbe soft and borderline metal ions such as HgII, HgI, PbII, PdII, CdII, AgI, and AuIII.(Yu et al., 2019) Similarly, MOFs possessing O-donor functions demonstrate an increased propensity for binding hard metal ions, including CrIII, AsIII, LnIII, ThIV, and UV(Ma et al., 2019). The focus has switched to creating extremely selective metal-binding ligands and their anchoring on the MOF surface due to the most recent methods of employing macrocyclic groups or chelating ligands with numerous donor sites.
| Physical properties | |||||
| MOF | S(m2g-1) | Dp(nm) | Morphology | Particle size | Referance |
| ZIF-8-mmen | 950 | 1.5 | Circular | 150 | (Wu, Ma, Li, et al., 2020) |
| CAU-7TATB | 330 | – | flat | – | (Wu, Ma, Wang, et al., 2020) |
| NH2 – SiO2@CuMOF | 200 | – | Octahedral | 450 | (Navarathna et al., 2020) |
| PCN-221 | 1100 | – | Spherical | 345 | (Chen et al., 2020) |
| MIL- 101(Cr)NH2 | 400 | – | Prismatic | 788 | (Zheng et al., 2019) |
| UiO-66-PEIs | 548 | 2.3 | Cubic | 14 | (Hasankola et al., 2020) |
| TMU-23 | 345 | 6.7 | Sheet | 43 | (Hasankola et al., 2020) |
| NH2 -ZIF-8 | 678 | 2.0 | Flat | 123 | (Lim et al., 2020) |
| UiO-66- (COOH)2 | 78 | – | Cubic | 876 | (Shao et al., 2019) |
| UiO-66- (COOH) | 445 | – | Spherical | 345 | (Meng et al., 2020) |
Given the grave risks that both humans and ecosystems face, it is imperative that organic and inorganic contaminants be accurately identified, removed from the environment, and continuously monitored. Because of the superior qualities of these coordination polymers, research on employing MOFs as an adsorption or sensing technique to eliminate hazardous metal ions has received more attention in recent years. In numerous environmental analytical applications, both original MOFs and hybridized and functionalized MOFs—which form composites with various materials—have been effectively used as sorbents. When compared to conventional porous materials, the UiO series of MOFs composites, represented by UiO-66, have shown exceptional potential as sorbents.
This is because of their known highest surface area, simple synthetic tunability, ultra-low density, unique cavity structure, and mechanical and thermal stability. We have tried to present some important and up-to-date research on the synthesis and uses of UiO-MOFs and their composites as cutting-edge and effective sorbents for the removal of heavy metal ions in this review. Additionally, a summary of the adsorption properties and mechanism of UiO-MOFs revealed that the majority of Freundlich isotherms, pseudo-second-order models, and Langmuir isotherms fit the experimental results quite well.
Thermodynamic analyses also demonstrated that metal ion adsorption is primarily spontaneous. But there are still many issues that need to be resolved, such as green synthesis techniques, commercial adsorption uses, the unique functionality of MOFs, and production and regeneration expenses.
The synthesis of new MOFs with higher chemical stability, good selectivity, super adsorption features, and better reusability is necessary for the future applications of MOFs as sorbents in separation science, even though UiO-MOFs and its composites have been the subject of extensive research in recent years and have demonstrated good adsorption effect. As a result, it is certain that MOFs will be made in an inexpensive, eco-friendly, and straightforward manner.
These selective sorbents’ ability to adsorb and remove extremely hazardous organic metals holds significant promise for advancing sustainable development in the years to come. Furthermore, issues including financial limitations, extensive sorbent regeneration, and excessive chemical use must be resolved when research moves from the laboratory to the pilot and industrial scales. The difficulties and directions show the interest in MOF chemistry’s ongoing expansion and promising future.